Abstract
INTRODUCTION
Patients affected by Philadelphia-negative chronic myeloproliferative neoplasms (MPNs) are considered at high risk of thrombo-haemorrhagic events, but the role of the platelet count in the assessment of the risk of vascular events is still controversial. A tight correlation was found between the platelet count and plasma sCD40L, which appears to be required for thrombus formation in vivo. However sCD40L is increased both in MPNs and reactive thrombocytosis. Intravascular aggregates of platelets and leukocytes, mediated by P-selectin and CD11b on the former and the latter, respectively, have been observed.
Both of this processes should imply a perpetual - and measurable - platelet activation. Several studies on platelet function have been already proposed, nevertheless the mechanisms through which platelets are able to trigger vascular events, are not yet adequately clarified.
A refined method for the determination of platelet activation appears to be the use of platelet PAC-1 antibody, able to identify the expression of the fibrinogen receptor of platelet glycoprotein IIb/IIIa. This expression is indeed unique in the process of platelet activation, and yet rarely analyzed. Moreover, since the platelet fibrinogen receptor exposure seems to be influenced by turbulence in blood flow, we have thought that its evaluation could provide important biological evidences to explain some clinical manifestations, such as microvascular disturbances.
METHODS
Blood samples from 40 consecutive MPNs patients who never received cytoreductive agents, were obtained. 28/40 patients were receiving a continuative antiplatelet prophylaxis with low dose aspirin (ASA, 75-100 mg) at the time of collection, while 12/40 of them were not on such therapy. Our aim was to verify the expression of platelet fibrinogen receptors (PFRs) in the two different groups of patients compared to healthy volunteers, using whole blood flow cytometry. In each experiment sodium citrate and heparin (positive control of platelet activation) tubes were collected from the same patient. Within 10 minutes from blood sampling, 5 ml of whole blood from each tube was incubated for 20 minutes at room temperature in the dark with saturating concentration of CD61 PerCP, CD62P PE and PAC-1 FITC. Positive control was also incubated with PAC-1 in the presence of Arg-Gly-Asp-Ser (RGDS) in order to test the specific antibody binding. Samples were fixed with paraformaldehyde 1% for 30 minutes at 4°C in the dark and analyzed on a flow cytometer.
RESULTS
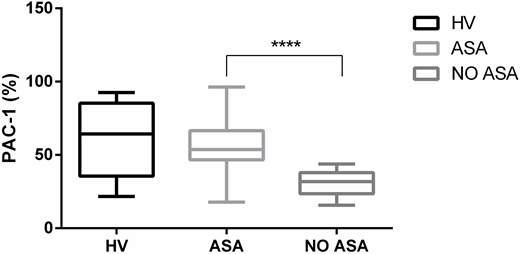
Surprisingly, we have been able to verify a very low PAC-1 binding to platelets in patients with MPNs not receiving cytoreduction nor antiplatelet agents (33%) if compared to that observed in healthy subjects (61%; p<0.0001). The use of aspirin seems conversely to restore the expression of platelet fibrinogen receptor, as PAC-1 binding capacity is comparable to that of healthy volunteers (56%). No difference was found with respect to the JAK2 Val617Phe mutation and its allele burden. Interestingly, by focusing on the group of patients under antiplatelet prophylaxis and with no history of thrombosis, it was found that subjects with persistent microcirculatory disorders show a higher PAC-1 binding capacity if compared to the asymptomatic ones (67% vs 52%, p= 0.04).
CONCLUSION
In untreated MPNs, a large amount of platelets are resting in a conformation that is unable to bind fibrinogen, as demonstrated by the low PAC-1 expression in cytofluorimetry. This lack of activity can be reversed by administering ASA, which is also known for its fibrinolytic and hypoprothrombinemic effects. We hypotesize that the hypercoagulable states observed in these patient could depend on a primarly plasma-driven impairment of fibrin turnover and thrombin generation. Further investigations are going to be promoted by our Group. PAC-1 could be at the same time a good marker of aspirin resistance in patients experiencing microcirculatory disorders.
Martinelli:Janssen: Consultancy; Jazz Pharmaceuticals: Consultancy; Ariad/Incyte: Consultancy; Pfizer: Consultancy, Speakers Bureau; Celgene: Consultancy, Speakers Bureau; Amgen: Consultancy; Abbvie: Consultancy; Roche: Consultancy; Novartis: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.